Safe and Effective? Part 2
Pfizer's vaccine trial data predicted millions of Serious Adverse Events
In my most recent post, “Safe and Effective?” I outlined the basic elements of the argument I use to present my position, that there is no evidence that the Covid-19 vaccine is either safe or effective, to other medical professionals. Summarizing the points from there:
Misinformation is coming from both sides of the issue
The vaccine trial results is the best (and perhaps only) body of data that both sides can agree is valid
Weighing the vaccine’s benefit in reducing the risk of Severe Covid with the risk of Serious Adverse Events (SAEs) incontrovertibly demonstrates a risk/benefit profile that does not favor authorization of the product
The product received authorization because of the peculiarly high background rate of serious adverse events in the population (placebo group)
The inexplicably large number of vaccine recipients that left the trial within 7 days of receiving their second dose proves that the investigators were unblinded
If the investigators were not blinded, the trial data is no longer valid
If the trial data is not valid, how then do we know that the Covid-19 vaccines are safe and effective? This is where we arrive at another impasse. The CDC continues to implore everyone to remain up to date with their vaccination status because it is the most effective method of staying safe.
They justify their position by citing studies that purportedly demonstrate that the product saves lives and hospitalizations from Covid-19. Without digging through the data we can say that these studies do not and cannot match the rigor of the randomized, placebo control study of the original Pfizer/BioNTech trial. There are an enormous number of observational studies that have been conducted since the vaccine campaign was launched over two years ago. Some demonstrate a modest Vaccine Effectiveness, some do not.
The public never hears about those that demonstrate that the effectiveness of the Covid-19 vaccines not only wanes, but goes negative. It is widely accepted that vaccine effectiveness wanes (why else has the public been urged to boost, reboost and most recently, bivalently boost?).
But negative effectiveness? Negative effectiveness means that the vaccinated have a greater risk of infection, hospitalization and death. Why would it turn negative?
I will not explore the possible answers to this question here. All that is required right now is to acknowledge that studies can be cherry-picked to support either side of this debate.
What can the Trial Data tell us?
Before we jettison the data that came from the original Pfizer trial, let's do some more digging. When we do, we will see that not only were the investigators unblinded, the majority of the vaccine recipients who were pulled from the trial likely suffered a SAE.
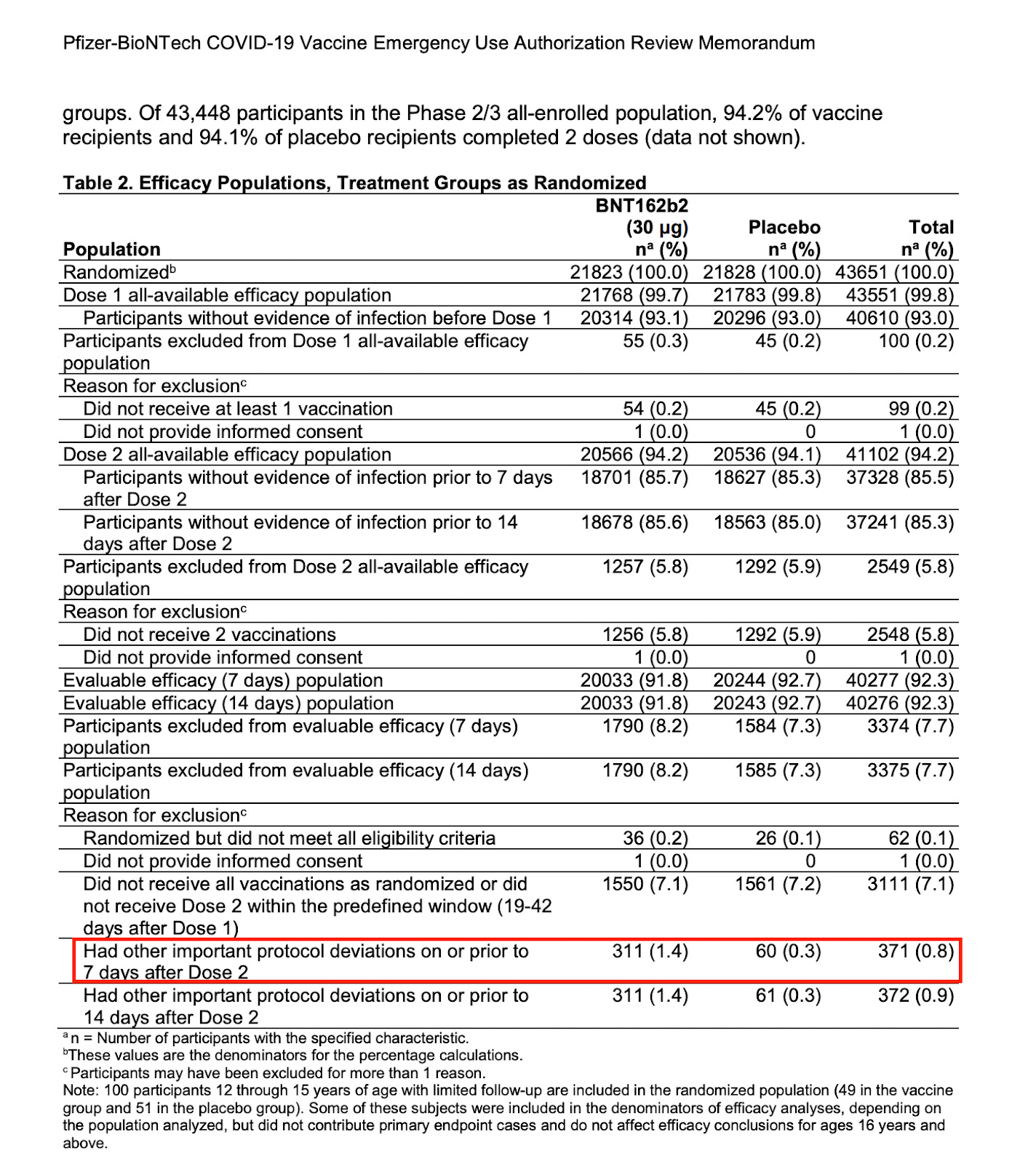
As I explained in “Safe and Effective?”, the unassailable proof of fraud appeared in a single table in a missive from Pfizer to the FDA written in support of their application for Emergency Use Authorization:
We see that 311 vaccine recipients were removed from the trial due to “important protocol deviations” while only 60 of placebo recipients were taken from the trial for similar reasons. There is only an infinitesimally small chance this could have happened by coincidence (less than 1 in 100,000). Add to it that a similar proportion were pulled from the pediatric trial months later at exactly the same point in the trial. Taken together, we can be certain that investigators knew which participants were receiving the vaccine and which were getting the placebo.
But what happened to those trial participants? Pfizer doesn’t say and the FDA didn’t ask. Note that these participants were removed from efficacy and safety evaluation after having received both doses and within seven days of receiving the second dose. Why would they be removed from consideration after having gone through the entire trial?
The most obvious explanation is that the vaccine recipients suffered a Serious Adverse Event (SAE). If they did, their outcome would have appeared as adverse events in the vaccinated column, but only if they remained in the trial. We can speculate that the nature of the adverse events were serious, and if that were the case, the safety of the vaccine would have been in question. That would be the motivation behind the selective exclusion of these participants from the trial.
There were 60 placebo recipients pulled at that point in the trial. It would be safe to assume that these 60 were not included because of true “protocol deviations” and not a serious injury. Thus, we would expect that the same number should have occurred in the vaccine arm as well. This means that about 250 participants who received the second dose may have had an untoward response to the vaccine that was intentionally hidden. Is this just pure speculation or do we have more to go on?
There were 20033 vaccine recipients that made it to the second dose. If approximately 250 of them had a Serious Adverse Event at that point, that would constitute a 1.25% rate of SAEs in excess of those in the placebo group.
Recall that the number of SAEs in both groups were nearly identical as reported (0.6% vs 0.5%). This was the reason vaccine risk, as defined by SAEs, was dismissed by our regulators.
If the actual risk of incurring an SAE is 1.25% (1 in 80) this would result in a staggering number of vaccine related injuries that would have occurred now that around 250 million people in the US have been vaccinated with the primary series.
Could this really be happening in our country? Our authorities say no, the risk of serious adverse events is exceedingly rare. The hundreds of thousands of reports of adverse events that have been accumulating in the Vaccine Adverse Event Reporting System (VAERS) are dismissed as “unverified” and “over reported”. True, they are unverified, but how do they know they are over reported?
They don’t. In fact, a CDC funded study from 2010, the Lazarus study, unequivocally proved that substantial underreporting occurs in VAERS. Lazarus found that only 1 in 10 to 1 in 100 vaccine adverse events get reported there.
Nevertheless, because VAERS is a passive reporting system (people have to take the initiative to report) and that reports could be fabricated, these numbers are not considered to be reliable.
However VAERS is not the only adverse event reporting system we have. V-Safe, an active surveillance system, has been in play throughout the Covid vaccine campaign. V-Safe enrolls vaccine recipients on a voluntary basis. Enrollees are contacted by phone immediately after they are vaccinated and at regular intervals afterwards.
V-Safe data was only recently made publicly available through a FOIA request submitted in 2021. What does it tell us?
The data set is enormous as over 10 million vaccine recipients chose to enroll. An interactive dashboard was constructed by Informed Consent Action Network (ICAN):
The key data point is indicated by the red arrow. 0.8 million out of approximately 10 million Vsafe participants reported requiring medical care following vaccination. That’s 8%. Could this be accurate?
ICAN is an organization that is denigrated as an anti-vax, agent of misinformation by vaccine advocates as Reuters points out in their coverage of this story. Then again, there is undeniable proof that the Pfizer vaccine trial was fraudulent and the FDA looked the other way. Who can be trusted?
The Reuters piece does bring up a valid point: the ICAN dashboard sums the number of people who sought medical attention during the full period of V-Safe surveillance, which was six months long. How can we know how many of those trips to the doctor or ER were due to the vaccine and not something else?
We are left with a lot of uncertainty. Why should we trust ICAN? Even if we did, we don’t know what percentage of the vaccinated sought medical care as a direct result of the vaccine because of the long period of reporting.
Let us look to the CDC. What do they have to say about the V-Safe data? Not surprisingly, very little. Though they had exclusive access to this data from the beginning of 2021, they never told the public about it. They only coughed it up because of legal action taken by ICAN and only after 15 months.
The CDC eventually delivered this enormous data set without any executive summary. This means that they either did not look at it or they did not want to admit that they did. However, the agency has offered the public their take on V-Safe surveillance data from the bivalent booster that was authorized in September, 2022.
In this Morbidity and Mortality Weekly Report (MMWR) published by the CDC in November, 2022, they report that within seven days of inoculation with the bivalent booster 0.8% of V-Safe enrollees reported seeking medical care.
The need for medical intervention constitutes a SAE. Recall that 1.25% of vaccine recipients that were excluded from the evaluable population were excluded within seven days of receipt of the second dose. It’s not unreasonable to assume that the safety of the original, monovalent vaccine approximates that of the newer bivalent vaccine. After all, that was the justification of authorizing this new product prior to the completion of any human trials.
We should be able to agree that there is ample evidence that
the vaccine trial investigators were not blinded,
this allowed them to selectively exclude participants from the evaluable population and
anywhere between 0.8 to 1.25% of vaccine recipients likely suffered Serious Adverse Events that were hidden from the trial results
If approximately 1% of vaccine recipients suffer serious adverse events there would be about 2.5 million SAEs in the US population alone. Why aren’t we hearing about them?
The answer is we are, but our authorities are not listening. At this time there are over a quarter million reports of SAEs in VAERS. This is about 10% of what the Pfizer trial would have predicted if those vaccine recipients who were excluded from the trial suffered a SAE. 10% is the upper bound of the percentage of vaccine injuries that get reported in VAERS according to the CDC sanctioned Lazarus study.
We now have a coherent explanation of what is really going on. The Pfizer trial results were fraudulent, and their vaccine is more than likely responsible for millions of Serious Adverse Events. Two different vaccine safety surveillance systems available to the CDC both confirm this number, more or less.
At this point I have only challenged the vaccine’s safety. Next I intend to take a critical look at the Pfizer vaccine’s purported efficacy.




Great article. The Pfizer trials, VAERS and V-Safe all point to Serious Adverse Events from the vaccines. It warrants pulling our collective heads out of the sand and investigating further. We need to wake up to the reality rather than trying to shut it out. The king is running around stark naked. Hallelujah for saying something about it!
The content/data in your article also aligns with the anecdotal evidence expressed through all the vaccine injury stories and sudden deaths in young people. I think the long-term side effects of having the spike protein migrate to different vital organs throughout the body is worse than we imagine. I desperately hope that I'm wrong. Most of my family and friends are fully vaxxed and boosted.